DIAGNÓSTICO
Diagnostic protocol for the determination of biomarkers in NSCLC
Given the clinical benefit of targeted therapies in lung cancer, the identification of molecular targets present in these patients is key 1
- Mandatory tests for all patients with NSCLC are: EGFR and BRAF mutations, ALK and ROS1 translocations, and PD-L1 expression. 3
- In 2019, the status of EGFR, ALK, ROS1, and PD-L1 in lung adenocarcinoma samples was analyzed in 91%, 80%, 56%, and 58% of patients, respectively.
3 - Susceptible histologic types for ALK determination include all adenocarcinomas, carcinomas with non-squamous histologic evidence, and squamous tumors in patients younger than 50 years and/or with low or no tobacco consumption (i.e., 15 pack-years).
1
SEAM AND SEOM CONSENSUS for the determination of biomarkers in advanced NSCLC
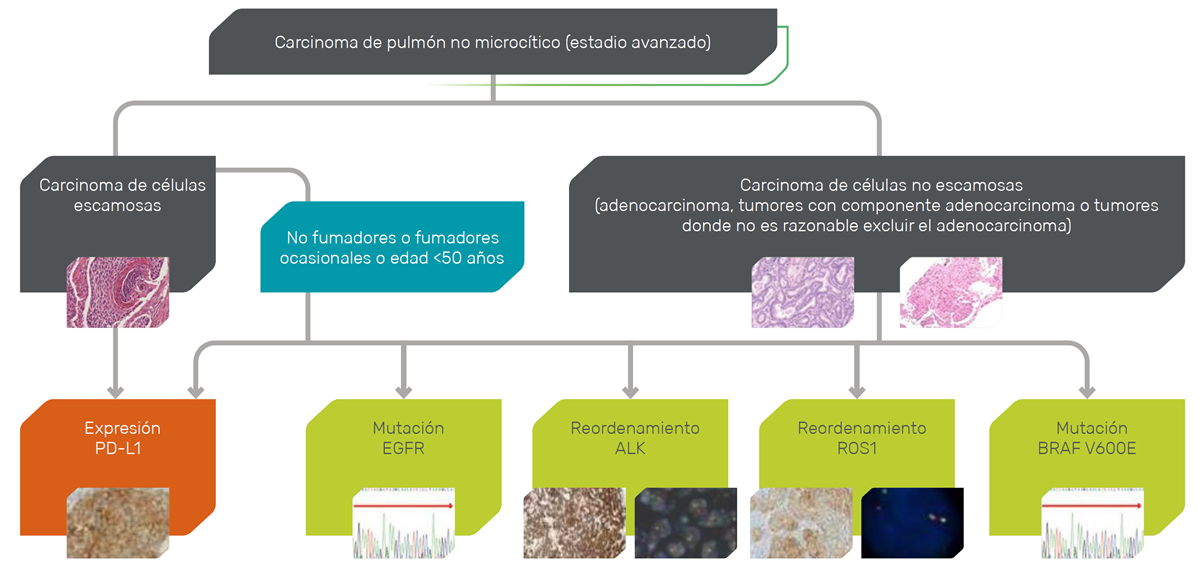
Image adapted from Figure 1 of Garrido P, et al. Clin Transl Oncol 2020.
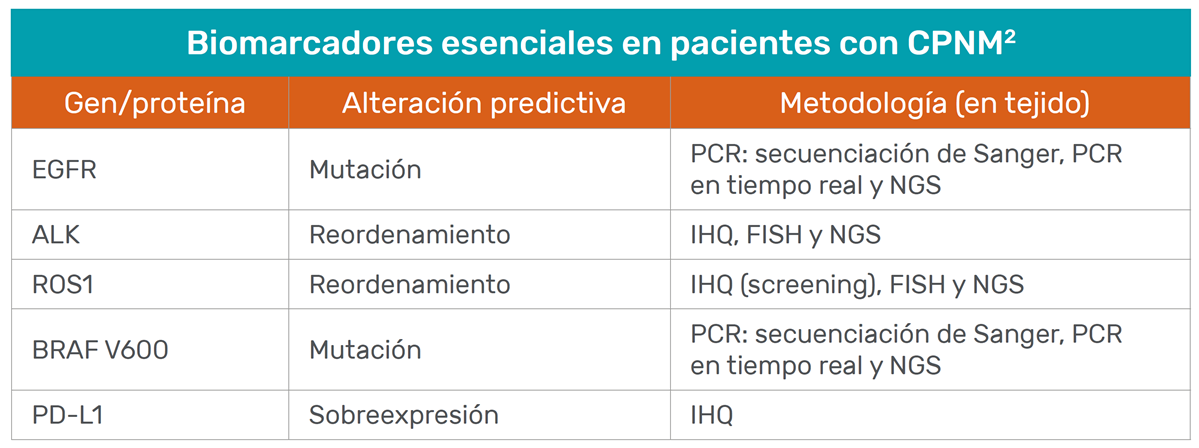
Table adapted from Table 1 of Garrido P, et al. Clin Transl Oncol 2020.
References
- Garrido P et al. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. 2020 Jul;22(7):989-1003.
- Diagnosis of Lung Cancer. Barcelona Clinic. [Internet]; [cited May 2024]. Available at: https://www.clinicbarcelona.org/asistencia/enfermedades/cancer-de-pulmon/diagnostico
- Remon J et al. Lung Cancer in Spain. J Thorac Oncol. 2021 Feb;16(2):197-204.

